Gut Microbiome and Allergic Diseases: A Revolutionary Paradigm for Prevention and Treatment
The human gastrointestinal tract harbors a complex ecosystem of trillions of microorganisms that collectively constitute the gut microbiome, a dynamic community whose influence extends far beyond digestive function to encompass fundamental aspects of immune system development, regulation, and response. This microbial ecosystem has emerged as a critical player in the pathogenesis of allergic diseases, representing a paradigm shift in our understanding of allergy development and offering unprecedented opportunities for prevention and therapeutic intervention.
The relationship between the gut microbiome and allergic diseases represents one of the most compelling examples of how environmental factors can shape human health outcomes through their effects on microbial communities. Over the past several decades, the dramatic increase in allergic disease prevalence in developed countries has coincided with significant changes in human microbial exposure patterns, antibiotic usage, dietary habits, and lifestyle factors that profoundly influence gut microbiome composition and function. This temporal correlation, supported by extensive mechanistic research, has established the gut microbiome as a central mediator of allergic disease susceptibility and severity.
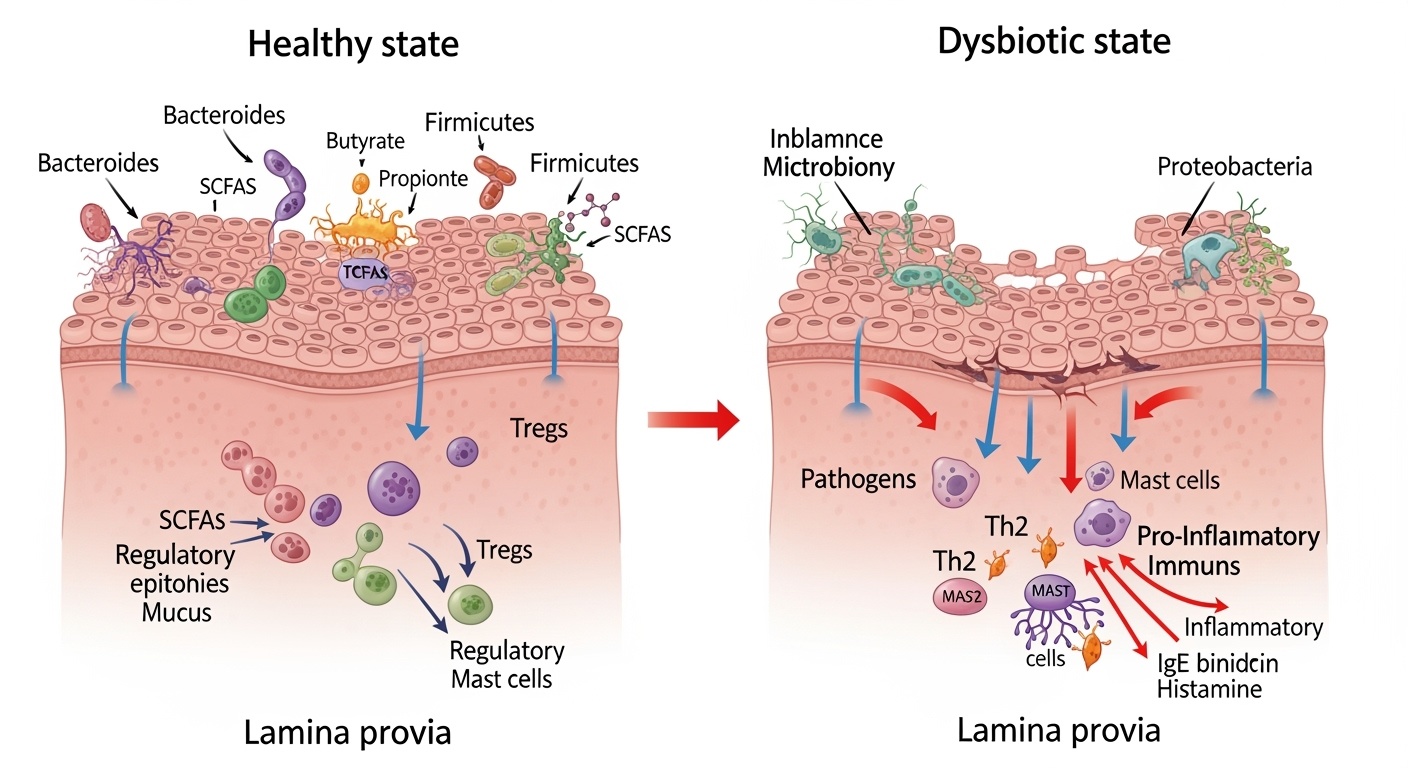
The concept of microbial dysbiosis, defined as an imbalance in the composition, diversity, or functional capacity of the gut microbiome, has become fundamental to understanding allergic disease pathogenesis. Unlike simple microbial infections caused by single pathogenic organisms, allergic diseases appear to result from complex alterations in entire microbial communities that disrupt the delicate balance between immune tolerance and reactivity. These disruptions can occur at multiple levels, from gross changes in major bacterial phyla to subtle alterations in specific microbial strains, metabolic pathways, and host-microbe interactions that collectively determine immune system behavior.
The therapeutic implications of the microbiome-allergy connection are profound and multifaceted, extending from preventive strategies that aim to optimize early-life microbiome development to targeted interventions that restore beneficial microbial functions in established allergic disease. The emergence of microbiome-based therapeutics represents a fundamental shift from symptom management toward addressing root causes of immune dysfunction, offering the potential for more effective and durable treatment outcomes while reducing dependence on traditional pharmacological approaches.
The Microbiome as an Immune System Educator
The gut microbiome serves as the primary educator of the human immune system, providing essential signals that guide immune cell development, differentiation, and functional programming throughout life. This educational process begins before birth and continues through critical developmental windows during infancy and early childhood, periods when the immune system exhibits remarkable plasticity and susceptibility to environmental influences. The quality and timing of microbial education during these formative periods appear to be crucial determinants of lifelong allergic disease susceptibility.
The mechanisms through which the gut microbiome educates the immune system involve complex networks of molecular interactions that encompass pattern recognition receptors, antimicrobial peptides, cytokine signaling cascades, and metabolic pathways. Commensal bacteria express pathogen-associated molecular patterns that are recognized by toll-like receptors and other pattern recognition receptors on immune cells, triggering signaling cascades that promote immune cell maturation and functional differentiation. These interactions are essential for establishing appropriate immune responses and maintaining the balance between protective immunity and tolerance to harmless antigens.
The development of regulatory T cells represents one of the most critical aspects of microbiome-mediated immune education. These specialized immune cells are responsible for maintaining immune tolerance and preventing excessive inflammatory responses to environmental antigens, including allergens. The gut microbiome provides essential signals for regulatory T cell development and function through multiple mechanisms, including the production of specific metabolites, direct cellular interactions, and the creation of an anti-inflammatory tissue environment that supports regulatory cell populations.
Microbial diversity plays a particularly important role in immune system education, with greater microbial diversity generally associated with more robust and balanced immune responses. High-diversity microbiomes expose the developing immune system to a broader range of microbial antigens and metabolic products, promoting the development of more sophisticated and nuanced immune responses. Conversely, low-diversity microbiomes may fail to provide adequate immune stimulation, leading to immune system immaturity and increased susceptibility to allergic sensitization.
The timing of microbial exposure appears to be critical for optimal immune education, with early-life exposures having particularly profound and lasting effects on immune system development. The concept of critical windows suggests that there are specific developmental periods during which microbial influences have maximal impact on immune programming. These windows may correspond to periods of rapid immune system development, gut barrier maturation, or other physiological processes that are particularly sensitive to microbial modulation.
Dysbiosis Patterns Across Allergic Disease Spectrum
The patterns of microbial dysbiosis observed in allergic diseases exhibit both common features and disease-specific characteristics that reflect the complex interplay between microbial communities, host genetics, environmental factors, and immune system dysfunction. Understanding these patterns is essential for developing targeted therapeutic interventions and predictive biomarkers for allergic disease risk and progression.
Food allergies are characterized by distinctive dysbiotic patterns that often manifest during the first year of life, coinciding with the critical period of oral tolerance development. Children with food allergies typically exhibit reduced microbial diversity, altered ratios of major bacterial phyla, and specific deficiencies in bacteria associated with immune tolerance induction. The reduction in Bifidobacterium species, particularly Bifidobacterium longum subspecies, appears to be a consistent finding across multiple food allergy cohorts and may represent a key mechanistic link between dysbiosis and failed oral tolerance development.
The metabolic consequences of food allergy-associated dysbiosis extend beyond simple taxonomic changes to encompass fundamental alterations in microbial metabolic capacity. Reduced capacity for complex carbohydrate fermentation, decreased production of short-chain fatty acids, and altered bile acid metabolism are common features of food allergy-associated dysbiosis. These metabolic deficits have direct implications for intestinal barrier function, immune cell development, and systemic inflammatory responses that contribute to allergic disease pathogenesis.
Respiratory allergies, including allergic rhinitis and asthma, are associated with dysbiotic patterns that may differ from those observed in food allergies while sharing certain common features. The gut-lung axis concept recognizes that respiratory allergic diseases can be influenced by gut microbiome composition through systemic immune effects, circulating microbial metabolites, and shared mucosal immune pathways. Children and adults with asthma often exhibit reduced gut microbial diversity, altered short-chain fatty acid production, and specific enrichments in pro-inflammatory bacterial taxa.
Atopic dermatitis represents another manifestation of the microbiome-allergy connection, with dysbiotic patterns that encompass both gut and skin microbial communities. The gut microbiome in atopic dermatitis patients often shows similar patterns to other allergic diseases, including reduced diversity and altered metabolic function. However, atopic dermatitis also involves distinctive changes in skin microbiome composition, with Staphylococcus aureus overgrowth being a particularly prominent feature that contributes to disease severity and treatment resistance.
The temporal dynamics of dysbiosis in allergic diseases reveal important insights into disease progression and potential therapeutic targets. Longitudinal studies have shown that dysbiotic patterns can precede clinical symptom development by months or years, suggesting that microbiome alterations may be causative rather than simply consequential. These findings support the potential for microbiome-based predictive biomarkers and early intervention strategies that could prevent allergic disease development.
Age-related changes in dysbiotic patterns reflect the dynamic nature of host-microbe interactions and the potential for microbiome-targeted interventions at different life stages. Early-life dysbiosis may be more amenable to correction through dietary interventions, probiotic supplementation, or environmental modifications, while established dysbiosis in older individuals may require more intensive interventions such as fecal microbiota transplantation or targeted antimicrobial therapies followed by microbiome restoration.
Metabolic Mediators of Microbiome-Immune Interactions
The gut microbiome influences allergic disease development and progression primarily through the production of bioactive metabolites that serve as molecular messengers between microbial communities and host immune cells. These metabolites represent the functional output of microbial metabolism and provide direct mechanistic links between microbiome composition and immune system behavior. Understanding these metabolic mediators is crucial for developing targeted therapeutic interventions and identifying biomarkers for allergic disease risk and treatment response.
Short-chain fatty acids represent the most extensively studied class of microbiome-derived metabolites with direct relevance to allergic disease pathogenesis. These metabolites, primarily acetate, propionate, and butyrate, are produced through bacterial fermentation of dietary fiber and other complex carbohydrates that escape digestion in the small intestine. The production of short-chain fatty acids requires specific bacterial enzymes and metabolic pathways that are enriched in certain bacterial taxa, particularly members of the Bacteroidetes and Firmicutes phyla.
Butyrate serves multiple roles in maintaining immune homeostasis and preventing allergic sensitization through its effects on intestinal epithelial cells, immune cell development, and systemic inflammatory responses. As the primary energy source for colonocytes, butyrate supports intestinal barrier integrity by promoting tight junction formation, mucus production, and epithelial cell turnover. These barrier-protective effects are essential for preventing inappropriate immune exposure to dietary antigens and environmental allergens that could trigger allergic sensitization.
The immunomodulatory effects of butyrate extend beyond barrier function to encompass direct effects on immune cell development and function. Butyrate serves as a histone deacetylase inhibitor, influencing gene expression patterns in immune cells and promoting the development of regulatory T cells while suppressing pro-inflammatory responses. These epigenetic effects provide a mechanism through which microbial metabolites can have lasting effects on immune system programming and allergic disease susceptibility.
Propionate exhibits complementary immunomodulatory effects that contribute to allergic disease prevention through distinct molecular mechanisms. This short-chain fatty acid can influence dendritic cell function, promoting the development of regulatory rather than inflammatory immune responses to environmental antigens. Propionate also affects systemic metabolic processes that may influence allergic disease risk through effects on adipose tissue inflammation, insulin sensitivity, and other metabolic parameters that are linked to immune function.
The bile acid metabolism pathway represents another important mechanism through which the gut microbiome influences allergic disease development. Specific bacterial taxa possess bile salt hydrolase enzymes that modify primary bile acids produced by the liver, generating secondary bile acids with distinct biological activities. These modified bile acids can influence immune cell function, intestinal barrier integrity, and systemic inflammatory responses through interactions with nuclear receptors and other signaling pathways.
Tryptophan metabolism represents a complex network of microbiome-host interactions that significantly influence immune function and allergic disease susceptibility. Bacterial tryptophan metabolism can generate various bioactive compounds, including indole derivatives that activate the aryl hydrocarbon receptor, a transcription factor that plays crucial roles in immune cell development and barrier function. The balance between different tryptophan metabolic pathways, influenced by microbiome composition and dietary factors, may determine whether tryptophan metabolism promotes immune tolerance or inflammatory responses.
Critical Windows of Microbiome Development and Allergy Risk
The concept of critical developmental windows has emerged as a fundamental principle in understanding how microbiome-immune interactions determine lifelong allergic disease susceptibility. These windows represent periods of heightened plasticity when environmental influences on microbiome composition and function can have profound and lasting effects on immune system programming. Identifying and characterizing these critical windows is essential for developing effective prevention strategies and optimizing the timing of microbiome-targeted interventions.
The prenatal period represents the earliest critical window for microbiome-immune programming, challenging the traditional view of the sterile womb and highlighting the importance of maternal microbiome health for offspring allergy risk. Maternal microbiome composition during pregnancy can influence fetal immune system development through multiple mechanisms, including the transfer of microbial metabolites across the placental barrier, the modulation of maternal immune responses that affect fetal development, and the preparation of the birth canal microbiome that will colonize the newborn during delivery.
Maternal antibiotic exposure during pregnancy has been associated with increased allergic disease risks in offspring, potentially through disruption of maternal microbiome communities and altered microbial metabolite production. These effects may be mediated through changes in maternal immune status, altered placental function, or modifications to the birth canal microbiome that affect neonatal colonization patterns. Understanding these prenatal influences provides opportunities for targeted interventions during pregnancy to optimize offspring allergy risk.
The birth process itself represents a critical transition point where the sterile fetal environment gives way to rapid microbial colonization that establishes the foundation for lifelong host-microbe relationships. The mode of delivery significantly influences early microbiome development, with cesarean section delivery associated with delayed colonization by beneficial bacteria and increased risks for allergic disease development. Vaginal delivery exposes newborns to maternal vaginal and fecal microbiomes, providing early inoculation with bacteria that are specifically adapted to the human gut environment.
The immediate postnatal period represents perhaps the most critical window for microbiome development and immune programming, as rapid microbial colonization occurs simultaneously with fundamental changes in immune system development and gut physiology. The first few days and weeks of life are characterized by dramatic changes in microbial diversity and composition as different bacterial taxa establish themselves in the neonatal gut environment. The success or failure of beneficial bacteria to establish stable populations during this period can have lasting consequences for immune system development and allergic disease risk.
Feeding patterns during early infancy represent one of the most important determinants of microbiome development during this critical window. Breastfeeding provides both direct microbial inoculation through breast milk microbiome and selective nutrition for beneficial bacteria through human milk oligosaccharides and other bioactive compounds. The unique composition of breast milk supports the establishment of Bifidobacterium-dominated microbiomes that are associated with reduced allergic disease risk and optimal immune system development.
The introduction of solid foods represents another critical transition point in microbiome development that coincides with the critical period for oral tolerance development. The timing, sequence, and composition of complementary foods can significantly influence microbiome maturation and immune system programming. Early introduction of allergenic foods, previously discouraged, is now recognized as potentially protective against food allergy development when introduced during the critical window of immune tolerance induction.
Antibiotic exposure during early childhood represents one of the most significant disruptions to normal microbiome development during critical windows. Early-life antibiotic use has been consistently associated with increased risks for allergic disease development across multiple cohort studies. The timing of antibiotic exposure appears to be particularly important, with exposures during the first year of life having the greatest impact on allergic disease risk. These effects may result from direct disruption of beneficial bacteria, altered metabolic function, or changes in microbial succession patterns that affect long-term microbiome stability.
Barrier Function and Allergic Sensitization
The intestinal barrier represents the largest interface between the human body and the external environment, serving as both a selective filter that allows nutrient absorption and a protective barrier that prevents inappropriate immune exposure to potentially harmful substances. The integrity and function of this barrier are critically dependent on the gut microbiome, which influences barrier development, maintenance, and repair through multiple complementary mechanisms. Disruption of barrier function represents a key mechanism linking microbiome dysbiosis to allergic disease development and provides important targets for therapeutic intervention.
The structural components of the intestinal barrier include the mucus layer, antimicrobial peptides, epithelial cells connected by tight junctions, and underlying immune cells that collectively create a dynamic and responsive barrier system. Each component is influenced by the gut microbiome through direct interactions and metabolite-mediated effects that maintain barrier integrity under normal conditions and promote rapid repair following injury or inflammation.
The mucus layer serves as the first line of defense against potentially harmful substances while providing a habitat for beneficial bacteria that contribute to barrier function. Mucus production is stimulated by specific bacterial taxa and their metabolites, particularly short-chain fatty acids that enhance goblet cell function and mucin gene expression. The composition and properties of mucus are also influenced by bacterial enzymes that can modify mucin structures, affecting mucus viscosity, antimicrobial properties, and bacterial adhesion characteristics.
Antimicrobial peptides represent another important component of barrier function that is regulated by microbiome-host interactions. These small proteins, including defensins, cathelicidins, and other antimicrobial molecules, are produced by epithelial cells and immune cells in response to microbial stimulation. The production of antimicrobial peptides requires appropriate microbial signals to maintain optimal levels that can control pathogenic bacteria without disrupting beneficial microbial communities.
Epithelial tight junctions represent the most selective component of the intestinal barrier, controlling paracellular permeability and preventing inappropriate translocation of antigens and microbes across the epithelial layer. Tight junction integrity is maintained through complex protein-protein interactions that are regulated by multiple signaling pathways, many of which are influenced by microbial metabolites and direct bacterial interactions with epithelial cells.
The concept of increased intestinal permeability, often referred to as “leaky gut syndrome,” has gained significant attention in relation to allergic disease development. Increased permeability can result from tight junction disruption, epithelial cell damage, or mucus layer depletion, leading to inappropriate immune exposure to dietary antigens, bacterial components, and other potentially allergenic substances. This increased exposure can trigger immune sensitization and promote the development of food allergies and other allergic diseases.
Microbiome dysbiosis can contribute to increased intestinal permeability through multiple mechanisms that affect different components of the barrier system. Reduced production of barrier-protective metabolites like short-chain fatty acids can compromise epithelial cell energy metabolism and tight junction integrity. Overgrowth of potentially pathogenic bacteria can produce toxins or enzymes that directly damage barrier components or trigger inflammatory responses that disrupt normal barrier function.
The systemic consequences of compromised barrier function extend beyond local intestinal effects to influence immune system behavior throughout the body. Increased translocation of bacterial lipopolysaccharides and other immunostimulatory molecules can trigger systemic inflammatory responses that promote allergic sensitization and exacerbate existing allergic diseases. These systemic effects help explain how gut microbiome dysbiosis can influence respiratory allergies, atopic dermatitis, and other allergic diseases that primarily affect non-gastrointestinal organs.
| Barrier Component | Microbiome Influence | Dysfunction in Allergies | Therapeutic Targets |
| Mucus Layer | SCFA-stimulated goblet cells | Reduced mucin production | Fiber supplementation, specific probiotics |
| Tight Junctions | Butyrate-mediated integrity | Increased permeability | Butyrate producers, barrier nutrients |
| Antimicrobial Peptides | Pattern recognition stimulation | Reduced antimicrobial activity | Immune-stimulating probiotics |
| Epithelial Turnover | Metabolite-fueled regeneration | Impaired repair responses | Microbiome restoration therapy |
Strain-Level Specificity in Allergy-Associated Dysbiosis
The recognition that bacterial strains within the same species can exhibit dramatically different functional properties has revolutionized our understanding of microbiome-allergy relationships and highlighted the importance of strain-level analysis in both research and clinical applications. This strain-level specificity means that the simple presence or absence of particular bacterial species may be less important than the specific strains present and their functional characteristics in determining allergic disease risk and treatment outcomes.
Bifidobacterium longum exemplifies the importance of strain-level differences in allergy-associated dysbiosis. While this species is generally considered beneficial for immune development and allergy prevention, specific strains within the species exhibit markedly different capacities for immunomodulation, metabolite production, and host interaction. Some strains of B. longum are highly effective at promoting regulatory T cell development and producing immunomodulatory compounds, while others may lack these beneficial properties or even exhibit pro-inflammatory characteristics under certain conditions.
The mechanisms underlying strain-level functional differences include variations in gene content, gene expression patterns, metabolic capabilities, and surface antigens that determine how bacteria interact with host cells and immune systems. Comparative genomic analysis has revealed substantial genetic diversity within bacterial species, with different strains possessing unique gene clusters that encode distinct metabolic pathways, virulence factors, or immunomodulatory compounds. These genetic differences translate into functional differences that can have profound effects on host health outcomes.
Ruminococcus gnavus represents another example of strain-level specificity in allergy-associated dysbiosis, with certain strains being enriched in children with allergic diseases while potentially beneficial strains of the same species are reduced. Allergy-associated strains of R. gnavus exhibit enhanced capacity for epithelial adhesion and gut colonization, potentially contributing to inflammatory responses and barrier dysfunction. These strains also show reduced capacity for complex carbohydrate degradation, contributing to the decreased short-chain fatty acid production observed in allergic individuals.
The identification of strain-specific markers associated with allergies has important implications for diagnostic applications and therapeutic development. Rather than relying on species-level identification, future microbiome-based diagnostics may need to incorporate strain-level analysis to accurately assess allergy risk and guide treatment decisions. This level of precision requires advanced analytical approaches including whole-genome sequencing, metagenomics, and functional profiling that can distinguish between closely related bacterial strains.
Therapeutic applications of strain-level specificity include the development of precision probiotics that target specific functional deficits in allergy-associated dysbiosis. Rather than using broad-spectrum probiotic formulations, future interventions may employ specific bacterial strains selected for their demonstrated efficacy in allergy prevention or treatment. This approach requires extensive strain characterization including safety testing, functional validation, and clinical evaluation to ensure both efficacy and safety.
The stability and persistence of therapeutic bacterial strains represent important considerations for strain-specific interventions. Some beneficial strains may be inherently unstable in dysbiotic gut environments or may be outcompeted by established pathogenic strains. Understanding the ecological factors that determine strain persistence is essential for developing effective interventions that can establish lasting changes in microbiome composition and function.
Environmental factors that influence strain-level selection and persistence include diet composition, pH levels, oxygen availability, antimicrobial exposure, and competition with other bacterial strains. These factors can create selective pressures that favor certain strains over others, potentially explaining why some individuals maintain beneficial strains while others develop allergy-associated dysbiosis despite similar environmental exposures.
Environmental Determinants of Microbiome-Allergy Interactions
The relationship between environmental factors, microbiome composition, and allergic disease development involves complex interactions that operate across multiple scales from individual exposures to population-level environmental changes. Understanding these environmental determinants is crucial for developing effective prevention strategies and identifying modifiable risk factors that could reduce the burden of allergic diseases at both individual and population levels.
The hygiene hypothesis has provided a foundational framework for understanding how reduced microbial exposure in modern environments contributes to increased allergic disease prevalence. However, the simple formulation of this hypothesis has evolved into a more nuanced understanding of how specific types of microbial exposures at critical developmental periods influence immune system programming and allergic disease risk. Not all microbial exposures are beneficial, and the timing, duration, and context of exposures appear to be crucial determinants of their effects on immune development.
Early-life antibiotic exposure represents one of the most consistently identified environmental risk factors for allergic disease development, with effects that appear to be mediated primarily through disruption of normal microbiome development. The magnitude of risk appears to be related to the timing, frequency, and spectrum of antibiotic exposure, with broad-spectrum antibiotics and multiple courses of treatment associated with the greatest risks. The critical importance of early-life antibiotic exposure has led to increased emphasis on antibiotic stewardship in pediatric populations and the development of guidelines for appropriate antibiotic use that balance infection treatment needs with long-term health consequences.
Mode of delivery represents another well-established environmental determinant of microbiome development and allergic disease risk. Cesarean section delivery bypasses the normal microbial colonization process that occurs during vaginal delivery, leading to delayed establishment of beneficial bacterial populations and altered microbiome succession patterns. The increasing rates of cesarean section delivery in many countries may contribute to population-level increases in allergic disease prevalence, highlighting the importance of optimizing delivery practices when medically appropriate.
Feeding practices during infancy represent modifiable environmental factors that significantly influence microbiome development and allergic disease risk. Breastfeeding provides both direct microbial inoculation and selective nutrition for beneficial bacteria, supporting the establishment of Bifidobacterium-dominated microbiomes associated with reduced allergy risk. The duration and exclusivity of breastfeeding appear to be important determinants of these protective effects, with longer breastfeeding durations generally associated with greater protection against allergic disease development.
The composition and timing of complementary food introduction represent additional dietary factors that influence microbiome development during critical windows of immune programming. Traditional recommendations for delayed introduction of allergenic foods have been replaced by evidence supporting early introduction during the critical window of tolerance development. However, the effects of early food introduction on allergy risk appear to be modulated by microbiome composition, suggesting that microbiome optimization may enhance the protective effects of early allergen exposure.
Agricultural exposures and rural living environments have been consistently associated with reduced allergic disease risk across multiple populations and geographical regions. These protective effects appear to be mediated through enhanced microbial diversity and exposure to specific bacterial taxa associated with agricultural environments. The mechanistic basis for these protective effects includes exposure to bacterial endotoxins that stimulate immune system maturation, consumption of unpasteurized dairy products that provide beneficial bacteria, and contact with farm animals that serve as sources of diverse microbial exposures.
Indoor environmental factors including pet ownership, household microbial diversity, and housing characteristics can significantly influence microbiome development and allergic disease risk. Pet ownership has generally been associated with reduced allergy risk, potentially through effects on household microbial diversity and early-life microbial exposures. However, the effects of pet ownership may depend on the timing of exposure, the types of pets, and individual genetic susceptibility factors that modify the relationship between pet exposure and allergy development.
Air pollution represents an environmental factor that can negatively impact both microbiome health and allergic disease development through multiple pathways. Exposure to particulate matter, nitrogen oxides, and other air pollutants can disrupt microbiome composition, promote inflammatory responses, and enhance allergic sensitization to environmental allergens. These effects may be particularly pronounced in urban environments where air pollution levels are elevated and opportunities for beneficial microbial exposures are limited.
Climate change and environmental degradation represent emerging threats to microbiome health and allergic disease prevention that operate at global scales. Changes in temperature, precipitation patterns, and extreme weather events can affect microbial communities in both human hosts and environmental reservoirs. Additionally, biodiversity loss and ecosystem disruption may reduce opportunities for beneficial microbial exposures that support healthy immune development, potentially contributing to continued increases in allergic disease prevalence.
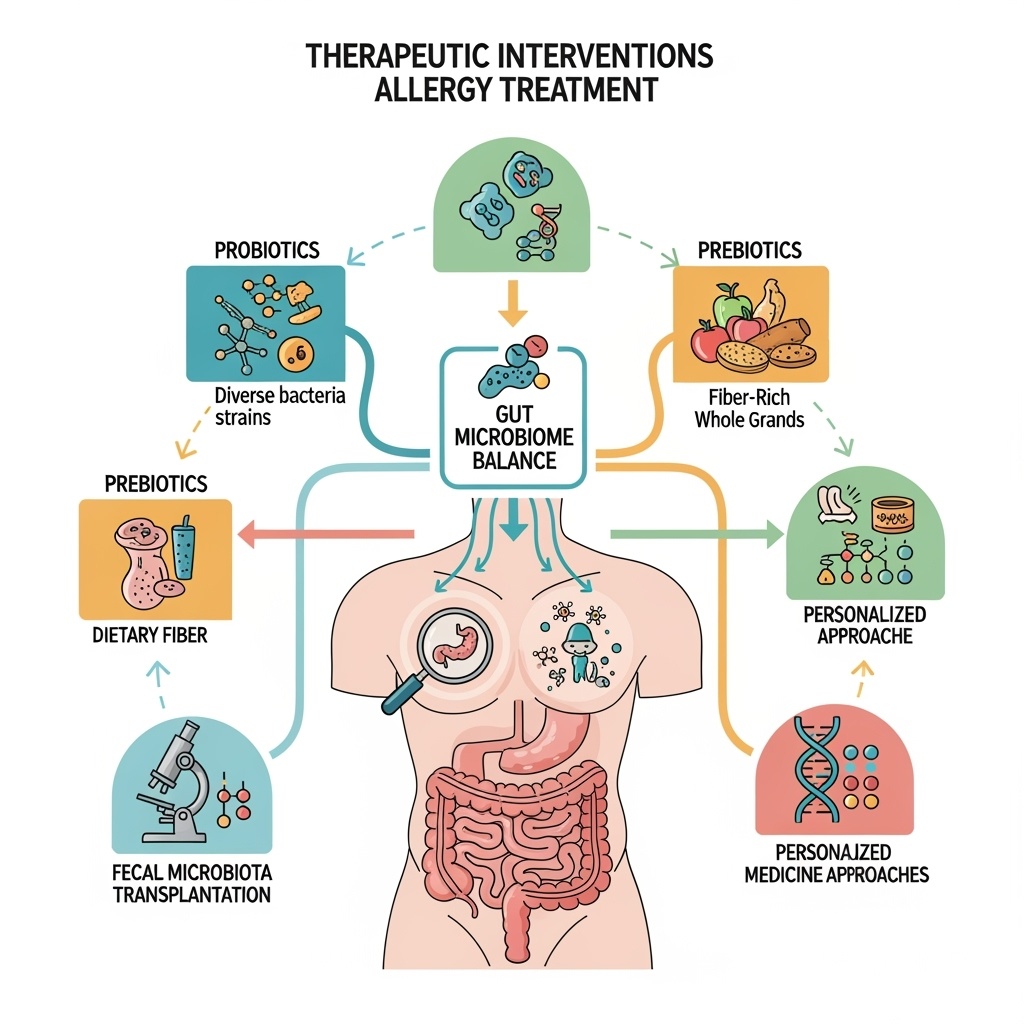
Therapeutic Interventions: From Probiotics to Precision Medicine
The translation of microbiome-allergy research into clinical applications has generated a diverse array of therapeutic approaches that range from simple dietary interventions to sophisticated personalized medicine strategies. These interventions target different aspects of microbiome function and can be applied at various stages of allergic disease development, from primary prevention in high-risk individuals to treatment of established allergic conditions.
Probiotic interventions represent the most widely studied and clinically available microbiome-based therapeutic approach for allergic diseases. Traditional probiotic formulations typically contain one or more bacterial strains, most commonly Lactobacillus or Bifidobacterium species, that are administered to modify gut microbiome composition and function. However, the clinical efficacy of probiotics for allergic disease prevention and treatment has shown considerable variability across studies, highlighting the importance of strain selection, dosing regimens, and patient population characteristics in determining treatment outcomes.
The development of next-generation probiotics represents an evolution beyond traditional approaches toward more targeted and mechanistically informed interventions. These advanced formulations may include bacterial strains specifically selected for their immunomodulatory properties, combinations of multiple strains designed to work synergistically, or genetically modified organisms engineered to produce specific therapeutic compounds. The selection of probiotic strains for allergic disease applications increasingly incorporates functional screening approaches that evaluate immunomodulatory capacity, metabolite production, and other relevant biological activities.
Prebiotic interventions focus on providing selective nutrition for beneficial bacteria rather than directly introducing new bacterial strains. These approaches typically involve dietary supplementation with specific carbohydrates, fiber types, or other compounds that preferentially support the growth of beneficial bacteria while limiting the expansion of potentially harmful species. The advantage of prebiotic approaches is that they work with existing microbial communities rather than attempting to introduce new organisms that may not establish stable populations in dysbiotic environments.
Synbiotic formulations combine probiotic and prebiotic components to provide both beneficial bacteria and their preferred nutritional substrates. This approach may enhance the establishment and persistence of therapeutic bacteria while supporting their metabolic activity and beneficial effects on host physiology. The development of effective synbiotic formulations requires careful consideration of bacterial strain characteristics, substrate preferences, and compatibility between different components.
Fecal microbiota transplantation represents the most comprehensive microbiome replacement therapy currently available, involving the transfer of entire microbial communities from healthy donors to recipients with dysbiotic conditions. While this approach has shown remarkable success in treating recurrent Clostridioides difficile infections, its application to allergic diseases is still largely experimental. The complexity of allergic disease pathogenesis and the potential risks associated with whole microbiome transfer require careful evaluation of safety and efficacy before widespread clinical implementation.
Targeted microbiome modulation approaches focus on selectively promoting or inhibiting specific bacterial populations without broadly disrupting entire microbial communities. These interventions may include narrow-spectrum antimicrobials that selectively target pathogenic species, bacteriophage therapy that specifically eliminates harmful bacteria, or engineered bacteria that can outcompete pathogenic strains while providing beneficial functions.
Dietary interventions represent accessible and potentially powerful approaches for microbiome modulation that can be implemented as standalone treatments or as adjuncts to other therapeutic approaches. High-fiber diets that promote short-chain fatty acid production have shown promise for allergic disease prevention and treatment. Specific dietary patterns such as the Mediterranean diet, which is rich in plant-based foods and beneficial fatty acids, may support microbiome health and reduce allergic disease risk through multiple complementary mechanisms.
Personalized microbiome therapy represents the ultimate goal of precision medicine approaches to allergic disease treatment. These interventions would be tailored to individual patients based on their specific microbiome profiles, genetic characteristics, environmental exposures, and clinical presentations. The development of personalized approaches requires sophisticated analytical tools for microbiome characterization, predictive algorithms for treatment selection, and flexible manufacturing systems for producing customized therapeutic formulations.
| Intervention Type | Mechanism of Action | Clinical Evidence | Implementation Challenges |
| Single-strain Probiotics | Direct bacterial supplementation | Mixed results, strain-dependent | Strain selection, persistence |
| Multi-strain Formulations | Synergistic bacterial interactions | Promising but limited data | Compatibility, quality control |
| Targeted Prebiotics | Selective nutrition for beneficial bacteria | Growing evidence for specific substrates | Individual variation in response |
| Fecal Microbiota Transplantation | Complete microbiome replacement | Limited data for allergies | Safety concerns, standardization |
| Precision Formulations | Personalized strain selection | Emerging research | Cost, analytical requirements |
Biomarkers and Predictive Models
The development of reliable biomarkers for microbiome-allergy relationships represents a critical need for advancing both research and clinical applications in this rapidly evolving field. These biomarkers must capture the complex interactions between microbial communities, host immune responses, and environmental factors while providing actionable information for clinical decision-making. The multi-dimensional nature of these relationships requires sophisticated analytical approaches that can integrate diverse types of biological data into clinically meaningful predictions.
Taxonomic biomarkers based on the relative abundance of specific bacterial taxa have provided initial insights into microbiome-allergy relationships but have shown limited clinical utility due to high inter-individual variability and the complex relationships between bacterial presence and functional activity. While certain taxa such as Bifidobacterium longum or Faecalibacterium prausnitzii show consistent associations with allergy protection across multiple studies, the predictive value of taxonomic markers alone appears insufficient for reliable clinical applications.
Functional biomarkers that capture microbial metabolic activity and host-microbe interactions may provide more robust and clinically relevant indicators of allergy risk and treatment response. These biomarkers include microbial gene expression profiles, metabolite concentrations in fecal or blood samples, and measures of microbial metabolic capacity such as short-chain fatty acid production potential. Functional biomarkers have the advantage of directly reflecting the biological processes that mediate microbiome effects on allergic disease development and progression.
Metabolomic biomarkers represent a particularly promising approach for capturing the functional output of microbiome-host interactions in a format that is amenable to clinical testing. Specific metabolites such as butyrate, propionate, tryptophan derivatives, and bile acid metabolites can be measured in biological samples using standardized analytical techniques and may provide direct indicators of microbiome function relevant to allergic disease risk. The development of targeted metabolomic panels for allergy prediction and monitoring represents an active area of research and development.
Immunological biomarkers that reflect the effects of microbiome composition on immune system function provide complementary information about the mechanistic pathways linking microbiome dysbiosis to allergic disease development. These biomarkers may include cytokine profiles, regulatory T cell frequencies, IgA production patterns, or other immune parameters that are influenced by microbiome composition and predict allergic disease outcomes.
Multi-omics integration approaches that combine taxonomic, functional, metabolomic, and immunological data may provide the most comprehensive and reliable biomarker signatures for clinical applications. These integrated approaches require sophisticated analytical methods including machine learning algorithms, network analysis, and other computational tools that can identify meaningful patterns in high-dimensional biological data. The development of clinically practical multi-omics biomarker panels represents a major challenge for translating research findings into routine clinical practice.
Longitudinal biomarker analysis is particularly important for understanding the dynamic nature of microbiome-allergy relationships and identifying critical transition points where interventions may be most effective. Single time-point measurements may miss important temporal patterns that are crucial for understanding disease development and predicting treatment responses. Longitudinal studies that track biomarker changes over time provide insights into causal relationships and optimal timing for therapeutic interventions.
Predictive modeling approaches that integrate biomarker data with clinical, genetic, and environmental factors may provide powerful tools for risk stratification and treatment selection. These models can incorporate machine learning algorithms that can identify complex non-linear relationships between multiple variables and generate probabilistic predictions about allergy development, disease progression, or treatment response. The validation and implementation of predictive models require large-scale prospective studies and careful attention to model performance across diverse populations.
The standardization of biomarker measurement techniques represents a critical requirement for translating research findings into clinical applications. Variability in sample collection, processing, storage, and analytical methods can significantly affect biomarker measurements and limit the reproducibility and clinical utility of research findings. The development of standardized protocols and reference materials for microbiome biomarker testing is essential for reliable clinical implementation.
Clinical validation of biomarker panels requires demonstrating their performance characteristics including sensitivity, specificity, positive and negative predictive values, and clinical utility in relevant patient populations. This validation process must address potential confounding factors, population-specific variations, and the relationship between biomarker values and clinically meaningful outcomes. The regulatory pathway for microbiome-based diagnostic tests is still evolving and may require novel approaches for evaluating complex biological signatures.
Future Directions and Clinical Translation
The future of microbiome-based approaches to allergic disease prevention and treatment lies in the successful integration of advancing scientific knowledge with practical clinical applications that can improve patient outcomes while remaining accessible and cost-effective. This integration requires continued progress in multiple areas including mechanistic understanding, therapeutic development, diagnostic capabilities, and healthcare delivery models that can effectively implement microbiome-based interventions.
The development of more sophisticated mechanistic models that can accurately predict the effects of specific microbiome interventions on allergic disease outcomes represents a key priority for advancing the field. These models must account for the complex interactions between microbial communities, host genetics, environmental factors, and immune system responses while providing actionable predictions for clinical decision-making. Advanced computational approaches including systems biology modeling, artificial intelligence, and digital twins of microbial ecosystems may provide powerful tools for developing these predictive capabilities.
Therapeutic development will likely move toward increasingly personalized approaches that tailor interventions to individual patient characteristics including microbiome profiles, genetic predispositions, environmental exposures, and clinical presentations. This personalization may involve custom probiotic formulations, targeted dietary recommendations, or combination therapies that address multiple aspects of microbiome dysfunction simultaneously. The development of flexible manufacturing systems and regulatory frameworks that can support personalized microbiome therapeutics represents a significant challenge for the field.
The integration of microbiome-based approaches with existing allergic disease treatments may provide opportunities for synergistic effects that improve overall treatment outcomes. Combination therapies that include microbiome interventions alongside traditional pharmacological treatments, allergen immunotherapy, or other established approaches may enhance treatment efficacy while reducing side effects or treatment duration. The development of rational combination therapy strategies requires careful study of potential interactions and optimization of treatment protocols.
Prevention strategies based on microbiome optimization during critical developmental windows may ultimately prove more effective and cost-efficient than treatment approaches for established allergic diseases. These prevention strategies could include prenatal interventions targeting maternal microbiome health, optimized delivery and early feeding practices, selective antibiotic stewardship, and environmental modifications that support healthy microbiome development. The implementation of population-level prevention strategies requires coordination between healthcare systems, public health agencies, and policy makers.
The development of point-of-care diagnostic tools that can rapidly assess microbiome status and guide treatment decisions represents an important goal for clinical translation. These tools must provide clinically actionable information in formats that are accessible to healthcare providers without specialized expertise in microbiome analysis. The integration of diagnostic capabilities with electronic health records and clinical decision support systems could facilitate the widespread adoption of microbiome-based approaches in routine clinical practice.
Educational initiatives targeting healthcare providers, patients, and the general public will be essential for successful implementation of microbiome-based approaches to allergic disease prevention and treatment. Healthcare providers need training in microbiome science, interpretation of microbiome-based diagnostic tests, and implementation of microbiome-targeted interventions. Patients and families need accessible information about the role of microbiome health in allergic disease development and practical guidance for implementing microbiome-supportive lifestyle changes.
Regulatory frameworks for microbiome-based therapeutics and diagnostics continue to evolve as regulatory agencies gain experience with these novel approaches. The development of appropriate regulatory pathways that ensure safety and efficacy while supporting innovation represents a critical need for the field. International harmonization of regulatory approaches may facilitate global development and implementation of microbiome-based interventions.
The economic evaluation of microbiome-based approaches will be important for demonstrating their value and supporting healthcare system adoption. Cost-effectiveness analyses must consider both direct healthcare costs and broader societal benefits including improved quality of life, reduced healthcare utilization, and prevention of chronic complications. The long-term economic benefits of prevention strategies may be particularly compelling but require sophisticated modeling approaches to quantify and communicate effectively.
Global health applications of microbiome-based approaches for allergic disease prevention may provide opportunities to address health disparities and improve outcomes in resource-limited settings. Simplified interventions that can be implemented without sophisticated laboratory infrastructure or extensive healthcare resources may have particular value for global health applications. The development of culturally appropriate and locally sustainable interventions requires careful consideration of local conditions, resources, and healthcare systems.
The ultimate success of microbiome-based approaches to allergic disease prevention and treatment will depend on their ability to provide meaningful improvements in patient outcomes while remaining practical, accessible, and cost-effective within diverse healthcare systems. This success requires continued collaboration between researchers, clinicians, industry partners, regulators, and patients to ensure that scientific advances are translated into real-world benefits for individuals and populations affected by allergic diseases. The revolutionary potential of microbiome-based approaches to transform allergic disease prevention and treatment represents one of the most exciting frontiers in modern medicine, offering hope for reducing the burden of these increasingly common conditions while advancing our understanding of the fundamental relationships between microbial communities and human health.
 allergix2.com
allergix2.com
